Why Your Nose Can Be a Pathfinder
When poking its nose into a hole, the rat is presented with one of two alternative smells. One smell tells the rat that food can be found behind the rat and to its left. The other smell tells it there is food to its right. After three weeks of training, the rats choose correctly more than 85 percent of the time. (Caption source: Science Nordic)
(Originally published by the Kavli Institute for Systems Neuroscience at NTNU)
April 17, 2014
When I was a child I used to sit in my grandfather’s workshop, playing with wood shavings. Freshly shaven wood has a distinct smell of childhood happiness, and whenever I get a whiff of that scent my brain immediately conjures up images of my grandfather at his working bench, the heat from the fireplace and the dog next to it.
Researchers at the Kavli Institute for Systems Neuroscience have discovered the process that connects smells to memories through an associative process where neural networks are linked through synchronized brain waves of 20-40 Hz. The results are published in this week´s edition of Nature.
We all know that smell is connected to memories, Kei Igarashi, lead author, explains. "We know that neurons in different brain regions need to oscillate in synchrony for these regions to speak effectively to each other. Still, the relationship between interregional coupling and formation of memory traces has remained poorly understood. So we designed a task to investigate how odour-place representation evolved in the entorhinal and hippocampal region, to figure out whether learning depends on coupling of oscillatory networks."
Smell Guides the Way in Maze
The researchers designed a maze for rats, where a rat would see a hole to poke its nose into. When poking into the hole, the rat was presented with one of two alternative smells. One smell told the rat that food would be found in the left food cup behind the rat. The other smell told it that there was food in the right cup. The rat would soon learn which smell would lead to a reward where. After three weeks of training, the rats chose correctly on more than 85% of the trials. In order to see what happened inside the brain during acquisition, 16–20 electrode pairs were inserted in the hippocampus and in different areas of the entorhinal cortex.
After the associations between smell and place were well established, the researchers could see a pattern of brain wave activity (the electrical signal from a large number of neurons) during retrieval.
Coherent Brain Activity Evolves with Learning
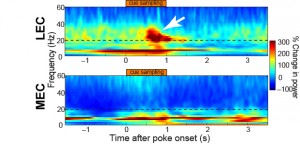
Immediately after the rat is exposed to the smell there is a burst in activity of 20–40 Hz waves in a specific connection between an area in the entorhinal cortex, lateral entorhinal cortex (LEC), and an area in the hippocampus, distal CA1 (dCA1), while a similar strong response was not observed in other connections, Igarashi explains.
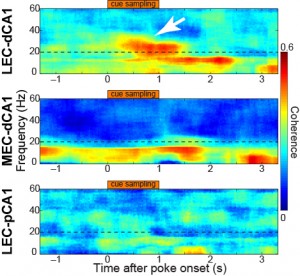
This coherence of 20–40 Hz activity in the LEC and dCA1 evolved in parallel with learning, with little coherence between these areas before training started. By the time the learning period was over, cells were phase locked to the oscillation and a large portion of the cells responded specifically to one or the other of the smell-odour pairs.
This is not the first time we observe that the brain uses synchronised wave activity to establish network connections, Edvard Moser, director of the Kavli Institute for Systems Neuroscience says. – Both during encoding and retrieval of declarative memories there is an interaction between these areas mediated through gamma and theta oscillations. However, this is the first study to relate the development of a specific band of oscillations to memory performance in the hippocampus. Together, the evidence is now piling up and pointing in the direction of cortical oscillations as a general mechanism for mediating interactions among functionally specialised neurons in distributed brain circuits.
So, there you have it – the signals from your nose translate and connect to memories in an orchestrated symphony of signals in your head. Each of these memories connects to a location, pinpointed on your inner map. So when you feel a wave of reminiscence triggered by a fragrance, think about how waves created this connection in the first place.
An Interview with Lead Author Kei Igarashi
It's a common experience: a smell triggers a memory of a time and place in your past. Earlier this month, a team of researchers from May-Britt and Edvard Moser's group at the Kavli Institute for Systems Neuroscience at the Norwegian University of Science and Technology (NTNU) discovered the process behind this phenomenon. The finding was reported in the journal Nature. After training rats to correctly identify the location of a food source based on an odor, the researchers identified a pattern of brain wave activity -- the electrical signal from a large number of neurons -- during the retrieval of this memory. The Kavli Institute researchers found these waves become more synchronized in two brain regions important for forming memories, the entorhinal cortex and CA1 of the hippocampus, after learning. The finding suggests that these two brain regions communicate more strongly after learning takes place.
We asked Kei Igarashi, first author of the Nature paper, to tell us about the discovery, and what it reveals about learning and memory.
Using mazes and rats to study behavior in animals is an established, standard method in scientific research. What was different about your use of it for this study?
We combined a behavior task that involves memory and brain signal recordings from regions that support memory. The task we used is an “odor-place association task,” which recruits the hippocampus and presumably the entorhinal cortex, brain regions important for for memory. We recorded brain signals from both of these regions when animals were performing this task, by attaching electrodes to the brain.
The Nature report suggests that rhythmic nerve cell activity, or oscillations, in the brain's cortex may be a general mechanism for orchestrating interactions among specialized neurons distributed in different parts of the brain. Besides smell and memory, what other brain functions may be mediated by such oscillations?
One of the most prominent advances on brain oscillations is work by Wolf Singer and colleagues in Germany. They found synchronization of brain oscillations in the visual cortex, and suggested its role for visual perception. Further work by Singer and Pascal Fries and other researchers suggests that visual attention, our ability to pay attention to a specific part inside the field of view, is mediated by synchronization of oscillations.
Your research findings add to the growing body of evidence that synchronization of brain waves enables circuits in different parts of the brain to communicate with each other. Under what conditions does this synchronization break down? What are the consequences?
It is known that patients with schizophrenia have a deficit in brain synchronization. On the other hand, patients with Parkinson’s disease are known to have excessive synchronization in motor areas in the brain. It would be important to have appropriate synchronizations for the brain to perform the right functions.
Impairment of a person's sense of smell has been linked to memory loss in neurodegenerative diseases such as Alzheimer's. What does your research tell us about that link?
The reason why loss of the sense of smell occurs in the early stage of Alzheimer’s disease is still largely unknown. It is known that the entorhinal cortex is one of the first areas in the brain to be affected in Alzheimer’s. So it’s possible that the loss of function in the entorhinal cortex leads to loss of the sense of smell as well as memory later. I think it is also possible that the synchronization we observed between the entorhinal cortex and the hippocampus is lost in Alzheimer’s disease.
What inspired you to study memory?
Before I moved to the Kavli institute at NTNU, I worked at the University of Tokyo and studied how brain regions for the sense of smell are organized. When I was observing brain sections under microscopes, I realized that a wide area inside the lateral entorhinal cortex receives a strong input of the sense of smell. I got more and more curious about the role of the entorhinal cortex, and that brought me to knock on the door of the Kavli Institute run by Edvard and May-Britt, which is one of the best labs studying the entorhinal cortex in the world.
Are there any smells that transport you to a certain time or place in your life?
I love hiking. Every time I smell an odor of pine tree, I remember pine resin that stuck to my hand on a hike when I was 15, together with beautiful scenery of snow mountains I saw at that time.