Improving Health Assessments with a Single Cell
(Originally published by the California Institute of Techniology)
May 24, 2011
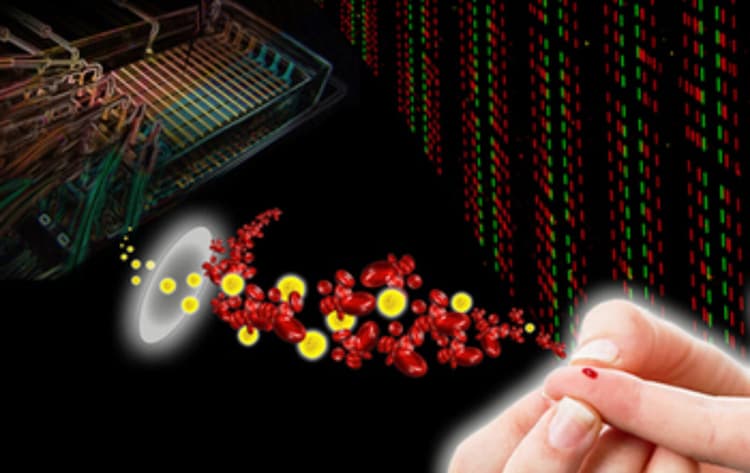
![Single Cell Barcode Chip (SCBC) enables personalized immune function monitoring: A small sample of a patient’s blood contains both white and red blood cells. The white blood cells are comprised of many different cell types, each of which perform various functions that together represent the activity of the immune system. Single immune cells are captured on the SCBC and their functional behavior read out by recording the levels of a dozen functional, secreted proteins for each captured cell.[Credit: Young Shik Shin/Caltech]](/uploads/images/image/nanoscience/1698-CT_Heath_SPOTLIGHT_medium.jpg)
There's a wealth of health information hiding in the human immune system. Accessing it, however, can be very challenging, as the many and complex roles that the immune system plays can mask the critical information that is relevant to addressing specific health issues. Now, research led by scientists from the California Institute of Technology (Caltech) has shown that a new generation of microchips developed by the team can quickly and inexpensively assess immune function by examining biomarkers—proteins that can reflect the response of the immune system to disease—from single cells.
The scientists reported on their advanced technology in the May 22 online issue of Nature Medicine.
"The technology permits us for the first time to quantitatively measure the levels of many functional proteins from single, rare immune cells," says James Heath, the Elizabeth W. Gilloon Professor, professor of chemistry at Caltech, board member of the Kavli Nanoscience Institute at Caltech and corresponding author of the study. "The functional proteins are the ones that are secreted by the cells, and they control biological processes such as cell replication and inflammation and, specific to our study, tumor killing."
In 2008, Heath—an expert in molecular electronics and personalized medicine—led the development of a "barcode chip" that, using just a pinprick's worth of blood, could measure the concentrations of dozens of proteins, including those that herald the presence of diseases like cancer and heart disease. This latest single-cell barcode chip (SCBC) device builds upon the success of that initial design, which is currently being utilized in diagnostic medical testing of certain cancer patients.
The researchers tested the chip by measuring a cancer patient's response to a type of cell-based immunotherapy designed to target and kill tumor cells. The only way to know if the therapy is doing its job is to measure many proteins at the same time from the individual cells that were targeting the tumor. The SCBC aced this test, generating readouts of a dozen secreted biomarkers—each of which represented a distinct cell function—and taking those readings from about a thousand single cells simultaneously.
The team was able to conduct a proof-of-concept study by looking at samples from a melanoma patient participating in the immunotherapy trials, and comparing those results to similar samples from three healthy subjects.
"This technology has the potential to be used routinely to monitor immune system performance," says Chao Ma, a graduate student in Heath's lab at Caltech's NanoSystems Biology Cancer Center and lead author of the Nature Medicine paper. "For example, it can be directly used to evaluate the effectiveness of certain classes of therapeutics, such as vaccines and other immunotherapies."
According to Ma, the technology is minimally invasive, cost-effective, and highly informative. The goal, he says, is to help physicians closely track the effectiveness of a therapy, and to rapidly alter or switch that therapy for the maximum benefit of the patient.
"The research fully demonstrates real-life clinical use of our revolutionary technology," Ma says.
The next step for the team will be to systematically apply the technology to clinical studies. The researchers have already begun to test the technology in additional patient populations, and to combine the SCBC with existing assays in order to get a more comprehensive picture of a therapy's efficacy.
In fact, the same study that showed the microchip's efficacy is already helping the researchers better evaluate the specific cancer immunotherapy trial, from which the patient in the study was drawn. "We are doing these same types of measurements on similar patients but at a significantly higher level of detail, and at many time points over the course of the cancer immunotherapy procedure," explains Heath. "It is helping us put together a 'movie' of the patient's immune system during the therapy, and it is providing us with some very surprising but also valuable insights into how the therapy works and how we might work with our UCLA colleagues to improve it."
“Application of this technology provides an unprecedented understanding of the human immune system by allowing an efficient and multiplexed functional readout of immune responses using limiting numbers of lymphocytes,” says Antoni Ribas, associate professor of medicine and physician who led the clinical trial portion of the study at UCLA's Jonsson Comprehensive Cancer Center.
The other Caltech authors of the Nature Medicine paper, "A clinical microchip for evaluation of single immune cells reveals high functional heterogeneity in phenotypically similar T cells," are postdoctoral scholar Qihui Shi; Rong Fan, former postdoctoral scholar; former graduate students Habib Ahmad and Gabriel Kwong; and Chao-Chao Liu, former undergraduate student. Begonya Comin-Andiux, assistant professor of surgery; Thinle Chodon, assistant researcher of medicine; Richard C. Koya, assistant professor of surgery; and Caius G. Radu, associate professor of medical and molecular pharmacology from UCLA's Jonsson Comprehensive Cancer Center also contributed to the study.
The work was funded by the National Cancer Institute, the Ivy Foundation, the Jean Perkins Foundation, the California Institute for Regenerative Medicine, the Caltech/UCLA Joint Center for Translational Medicine, the Melanoma Research Alliance, and the National Institutes of Health.