Exploring the Brain’s Plasticity with Nematodes
by Sally Johnson
Manipulating a tiny worm’s genes at the DNA level offers clues about how the human brain responds to injuries
The Author
The Researcher
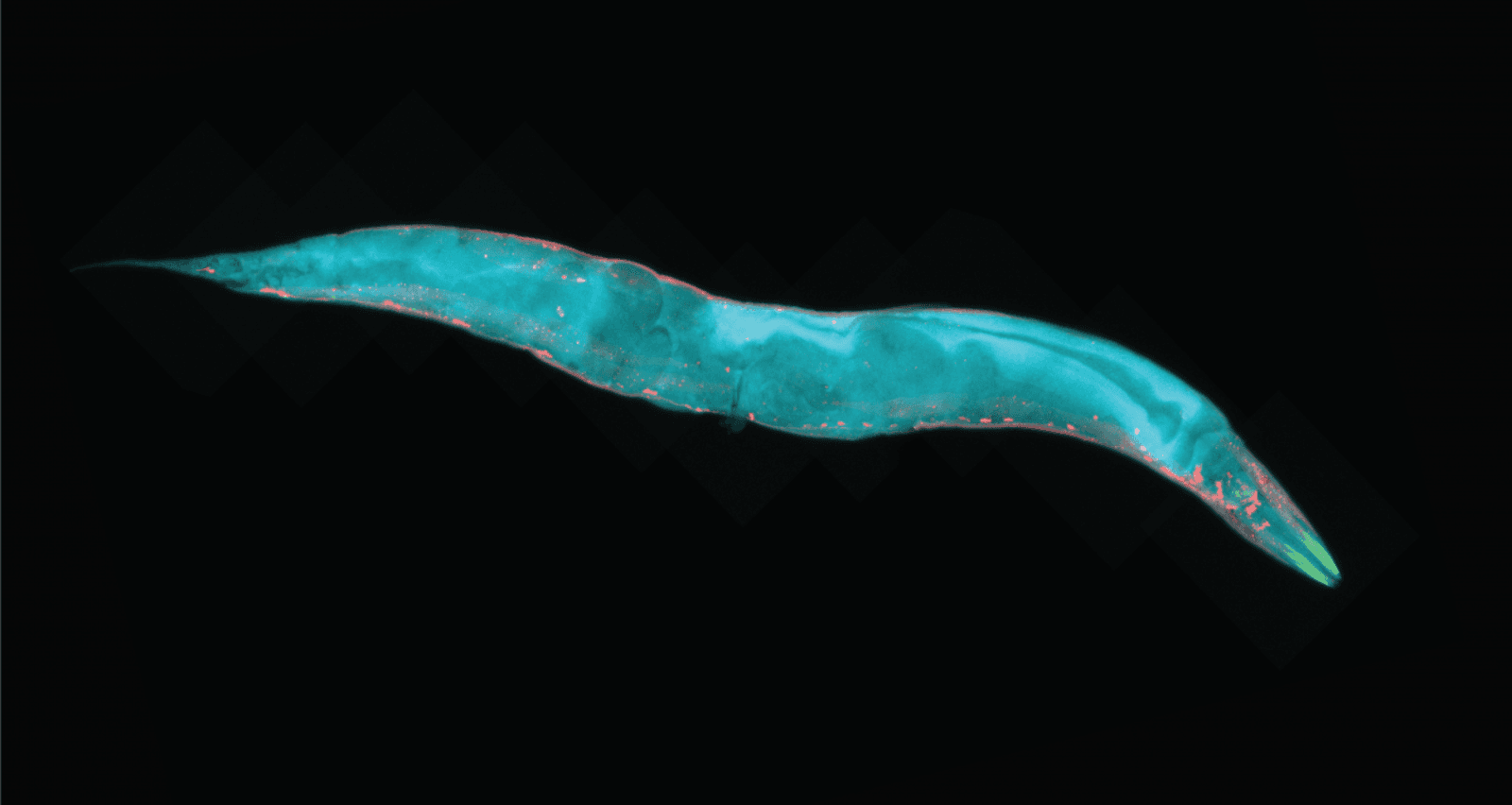
C. elegans, a.k.a. a nematode or tiny roundworm, is helping Yishi Jin, co-director of the Kavli Institute for Brain and Mind, as well as a distinguished professor of neurobiology at UC San Diego, uncover secrets of how brains work.
Jin studies C. elegans to explore molecular genetic mechanisms of synapse formation, neuron circuit regulation, and nerve regeneration. “My heart is in genetics, and C. elegans offers many ways to do genetic screening to discover molecular genetic mechanisms for how nervous systems are developed, and to learn about how neurons function and circuits work,” she says.
Why focus on C. elegans? It’s the only organism whose connectome, meaning the entire brain's structural and functional connections between cells, is known. This provides essentially a map of all neural connections within their brain.
“C. elegans is wonderful for anyone who wants to explore how genetic mutations affect brain function,” Jin says. “It has many behaviors—from sensory to motor neurons to neural networks. It’s also enabled many technologies: connectomics, genome sequencing, and optogenetics (using light to control genetically engineered brain cells to respond to specific wavelengths).”
Jin manipulates genes at the DNA level to gain a better understanding of neuroplasticity processes—the ability of the brain’s neural networks to change or grow through reorganization. For her, this started with an early interest in exploring the mechanisms of synapse formation via genetic approaches. Synapses are important because they allow neurons to pass electrical or chemical signals to other neurons or cells.
“We now have a pretty good understanding of the synaptic components, so we’re currently working on their plasticity aspect—neurons’ connectivities can change during development or under injury,” she says. “Two aspects of plasticity that I’m focusing on are developmental and trauma-induced.”
Since C. elegans is so tiny, 1 millimeter in length at its largest, it requires a microscope to label all of its synapses and neuromorphology (shape and connectivity) with fluorescent proteins.
“To impose injury to C. elegans neurons, we attach an ultrafast laser to a microscope and then oblate a single synapse and transect axons, which is what we call ‘trauma’ to its neurons, in many ways creating a situation to mimic a crude spinal cord injury where the axons get severed or damaged due to any kind of operation or accident—essentially nerve damage,” Jin explains. “In C. elegans, this damage is done via laser.”
Using transgenic (genetic engineering) or classical genetics to increase or decrease the gene activity, Jin then asks while manipulating genes: how do those traumatized axons respond? “We’ve done this for more than 2,000 genes,” she says.
Neurons usually respond by forming growth cones and extending their axons following injury. “Worms are like humans when their axons are damaged,” Jin points out. “It triggers a stress response, which involves growing their axons aimlessly as if trying to repair the damage. If we can find the gene that’s really critical for initiating this response, we can also find the gene that’s inhibiting it. When you remove inhibitors, the axons respond much faster.”
And what this means mechanistically, which is a passion of Jin’s, is that fundamental research on the mechanisms for neuronal cell biology—things regulating genes—can end up revealing drug inhibitors. “We’re working on genes that have homologs in humans, and through dissecting the function of these genes in C. elegans, we hope to identify the key controller that can be used for drug development.”
Jin’s philosophy is to identify the interesting biological context and use genetic logic to deduce how genes interact and figure out how genetic mutations alter this genetic interaction.
At the Kavli Institute for Brain and Mind, Jin is seeing a rapidly increasing effort toward interdisciplinary work—including a new partnership with UC San Diego’s engineering school. “Gabriel Silva, who used to be very much a brain scientist but who is now more of a computation neuroscientist and bioengineer, is an associate director here,” she says. “This is helping us really expand into engineering, making connections to brain and mind.”