Interneuron Networks Control Distinct Elements of The Brain´s Positioning System
(Originally published by the Kavli Institute for Systems Neuroscience)
October 19, 2017
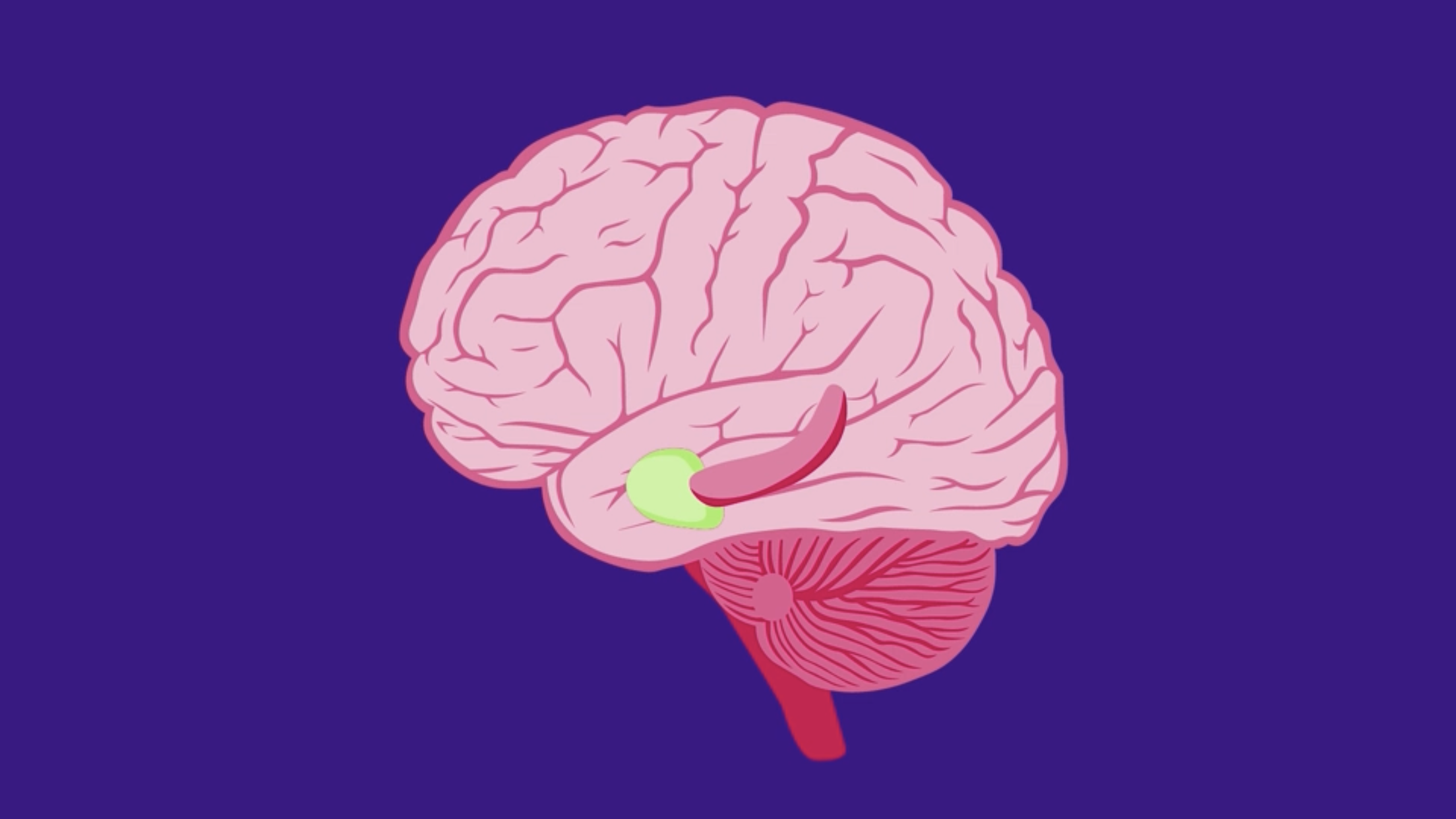
Navigating the busy city streets, do you ever reflect on how your brain is able to guide you in the right direction? Deep inside your brain, scientists have found a neural network continuously measuring and calculating your body’s movements and position in your surroundings, dynamically updating its calculations according to the passing landscape.
Scientists have until recently not known much about the specific circuits and mechanisms that allow for the precise control of self-positioning in this network. Recently, a group of scientists at the Kavli Institute for Systems Neuroscience in Trondheim, set out to uncover the neurons that shape and regulate large-scale activity in this network. They identified two distinct classes of interneurons that orchestrate independent cell classes of the positioning system.
The Brain's GPS
Unravelling how this neural circuit works, begins with understanding its component parts and the roles they play in navigation and memory. The brain’s headquarters for self-positioning sits in an area called the medial entorhinal cortex. Scientists have discovered a collection of specialized cell types in this area that are receptive to different features of your local space, like distances and borders.
The first cell type to be discovered back in 2005 was the grid cell. Grid cells are neurons that provide your brain with a GPS-like function and produce a metric for space. Grid cells use your own motion to map your location in the environment. By firing at fixed locations in the environment, each cell produces a regular hexagonal pattern that tiles the available space. In order to calculate position from movement, grid cells need information about speed. Speed cells measure your running speed through the environment. Another type of neurons, border cells, express your proximity to geometric borders in the landscape. Head direction cells track your orientation relative to landmarks in your environment. Researchers have also observed cells that respond to location but do not form a grid pattern. These cells are known as aperiodic cells.
– Grid cells need to be connected to other grid cells with similar firing pattern in order to provide the brain with exact data for localization and self-orientation, explains Edvard Moser, co-director of the Kavli Institute for Systems Neuroscience.
– The periodic nature of the grid pattern suggests a high level of coordination across functionally connected ensembles of grid cells. However, most grid cells in the medial entorhinal layer II area (MEC II) only connect to and communicate via interneurons, says Moser.
This led the researchers Chenglin Miao, Qichen Cao, May-Britt Moser and Edvard Moser to ask whether these interneurons could play an essential role in the production of the stable spatial grid pattern.
Interneurons - Gatekeepers of Neural Circuits
The two most common categories of neurons in the brain are excitatory and inhibitory neurons. Grid cells and other neurons sensitive to features of space are all excitatory neurons. Interneurons, providing connectional pathways between ensembles of neurons, are inhibitory. They are often thought of as the breaks of the brain. When an interneuron is active, it usually inhibits activity in the excitatory neurons that it connects to. When interneurons are quiet, excitatory neurons fire.
Operating by Switch or Filter
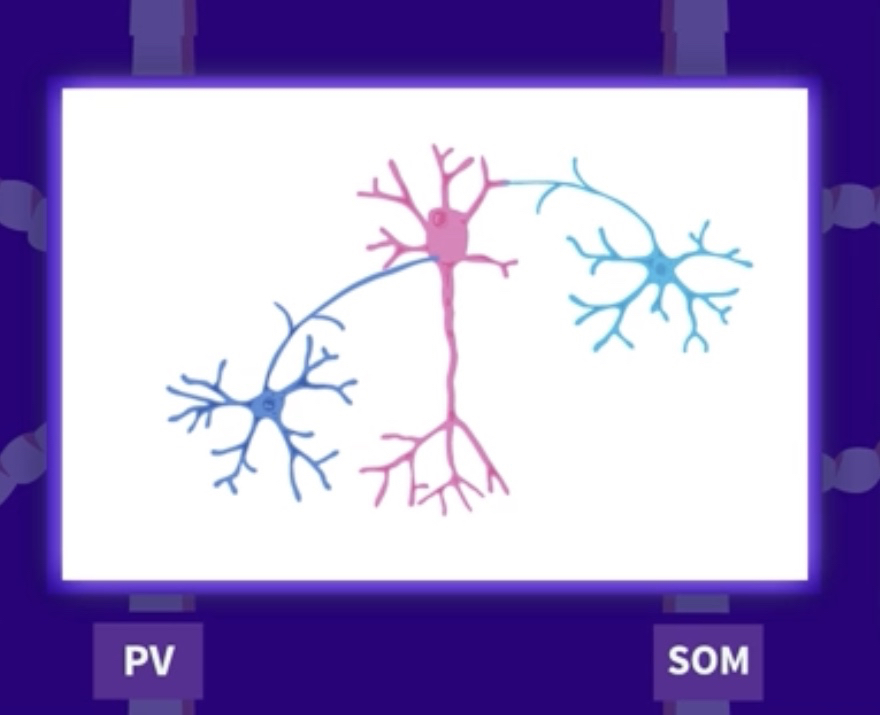
There are two major classes of interneurons in the medial entorhinal cortex: those that contain parvalbumin and those that contain somatostatin. These two classes of interneurons bind differently to ensembles of excitatory space cells. Parvalbumin (PV) interneurons bind synaptically directly to the soma of their goal cells, providing a switch-like function for directly turning on or off the activity of the excitatory cells they regulate. Somatostatin (SOM) interneurons, by contrast, bind to the dendrites of their goal cells, selectively gating excitatory input signals from different sources, thereby changing by arithmetic their relative contributions to the output of the excitatory goal cell.
The Experiment
The group of researchers at Kavli Institute for Systems Neuroscience wanted to find out how the inhibitory interneurons of the parvalbumin and somatostatin groups exert control over the circuit of spatial cells in the medial entorhinal cortex. To test this, they set up an experiment measuring activity from the various space-modulated, excitatory cell types in freely behaving mice.
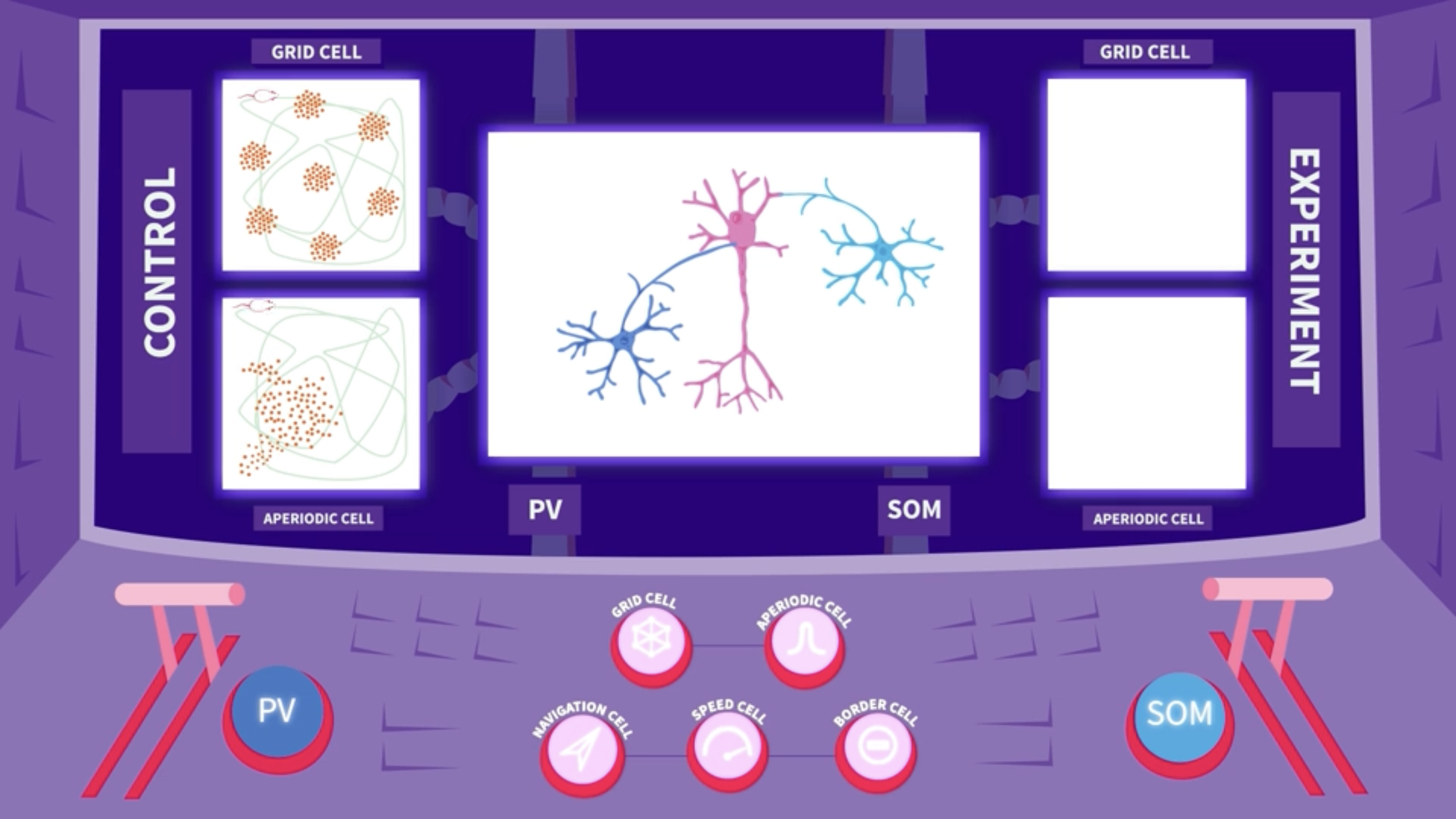
In the baseline state, before any intervention, all excitatory cells responded to spatial features in their normal manner: Signals from the grid cell formed a hexagonal pattern covering the surface of the box, while spikes from the aperiodic cell drew up one or several larger fields with no particular pattern.
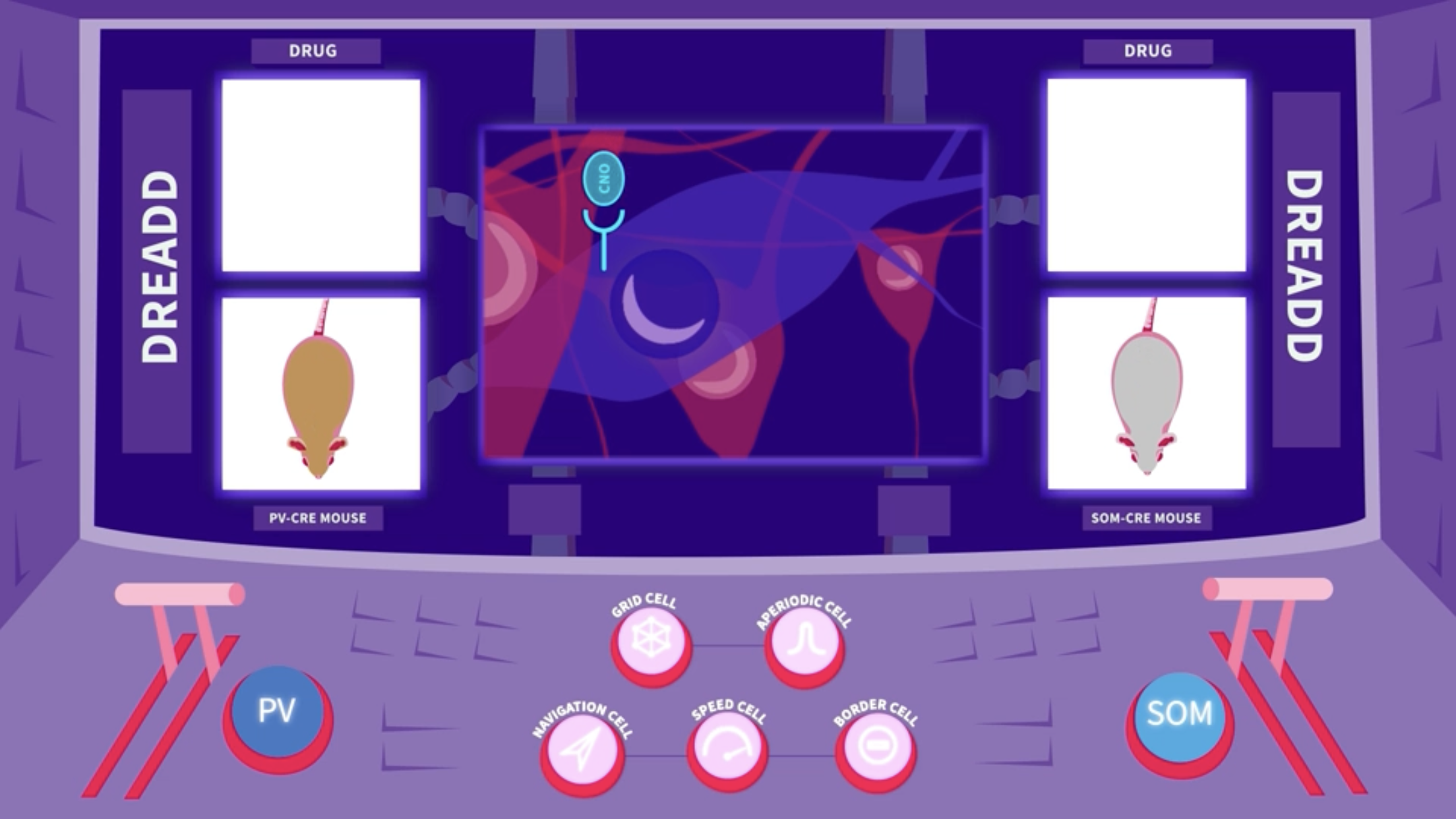
The researchers then used a pharmacogenetics tool to selectively silence the activity of either parvalbumin (PV) or somatostatin (SOM) interneurons. To target the two subgroups selectively, they used two groups of PV-Cre and SOM-Cre transgenic mice. A Cre-dependent AAV virus was injected into the medial entorhinal cortex of each group. This virus carried the gene for an artificial receptor, a DREADD. The Cre dependence ensured that the receptor was expressed exclusively in parvalbumin (PV) or somatostatin (SOM) interneurons, depending on the mouse line. When the drug CNO was introduced into the body, it bound to the DREADD receptors only, and caused selective silencing of either parvalbumin (PV) or somatostatin (SOM) cells for a period of hours.
Silencing the parvalbumin (PV) interneuron altered the grid cells´ behaviour, producing random noise that almost concealed the hexagonal grid signal. The signal from speed cells was also reduced, while aperiodic cells remained unaffected.
Silencing the somatostatin (SOM) interneurons, by contrast, did not affect the grid pattern. Instead, the firing location of the aperiodic cells became more dispersed and less confined.
Conclusions
- The experiments reveal that parvalbumin (PV) interneurons interact in a precise manner with grid and speed cells to produce the grid pattern that allows spatial mapping, while somatostatin (SOM) interneurons modulate aperiodic cells, Miao Chenglin explains.
- There was no recorded overlap between the two. This suggests that the medial entorhinal cortex is being managed by two distinct interneuronal networks that operate quite independently of each other.
- This was quite a surprise, says Edvard Moser. In other brain regions where parvalbumin and somatostatin interneurons have been investigated, such as the amygdala, they interact quite extensively, so we did not expect them to control distinct cell types so independently.
- The independent modulation of aperiodic cells by somatostatin (SOM) interneurons, points to these excitatory cells as a distinct category of spatially tuned cells, points out Qichen Cao.
Authors: Chenglin Miao, Qichen Cao, May-Britt Moser and Edvard I. Moser
Kavli Institute for Systems Neuroscience and Centre for Neural Computation, NTNU
Read the full article: Parvalbumin and somatostatin interneurons control different space-coding networks
in the medial entorhinal cortex published in Cell, 19 October 2017