New Sensor Sweats It Out
by Alan S. Brown
A new type of wearable sensor could let doctors, apps, and researchers monitor our health by measuring the chemicals within our bodies
The Author
The Researcher
Dozens of fitness devices—from Fitbits and Apple Watches to smartphone apps—measure physical signals, like heartbeat, temperature, and steps all day long. The data lets users assess their health and perhaps even learn about an impending medical problem.
Still, when it comes to really diagnosing illness, fitness devices are nowhere near as powerful as laboratory tests. These examine samples of blood, urine, and saliva for the presence of biomarkers, molecules that might show the presence of a serious disease. Unfortunately, taking these tests is time-consuming and usually involves complex medical equipment.
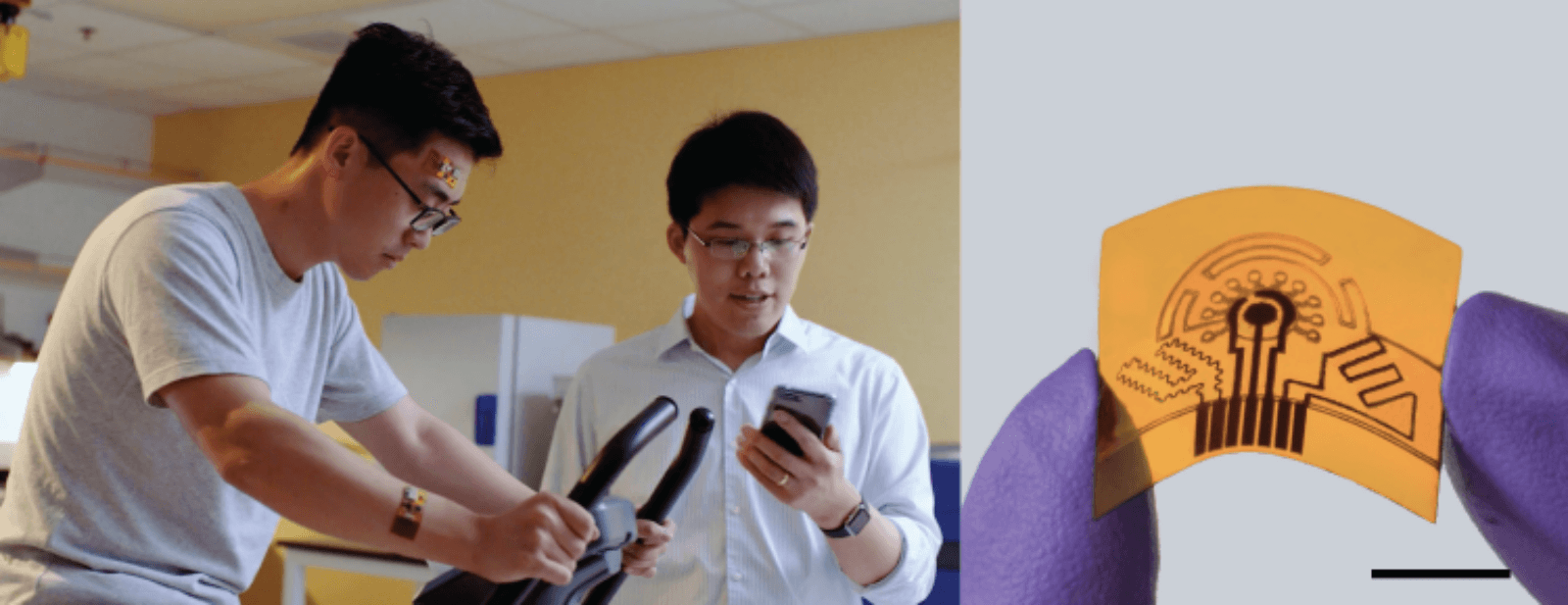
Wei Gao, an assistant professor of medical engineering and a member of the Kavli Nanoscience Institute at Caltech, is trying to bridge the gap between wearables and lab testing with a wearable array of sensors that analyzes sweat.
Why sweat? It turns out that sweat contains many biomarkers produced by the body, but in such trace amounts that even laboratory equipment cannot always measure them. Gao gets around that problem by using nanotechnology to identify key biomarkers for conditions that range from dehydration and low electrolytes to diabetes, gout, stress—and how the body is responding to medication. The system even uses sweat for power and, unlike laboratory tests, provides diagnostic data around the clock.
Sweating it out
“A sensor that collects information from the body at the molecular level all day long will change our lives by making personalized healthcare possible,” Gao said.
“When people get a blood test, they get only the data from the time when the blood was drawn. Yet many biomarkers fluctuate during the day. Cortisol, which indicates stress, is an example. It is high in the morning and low in the afternoon. We have to see how it changes from hour to hour to know the full picture,” he said.
While sweat is readily available 24/7, even when we are not exercising, its biomarkers often appear in concentrations measured in the trillionths of grams, or picograms.
To measure such minute amounts, Gao developed an innovative way to make his sensor. This starts with a piece of nylon plastic. Gao then uses a carbon dioxide laser to engrave channels into it. Then he turns up the laser’s power, so it is hot enough to burn off all the nylon’s atoms except for the carbon. By carefully controlling conditions while he does this, he produces a carbon flower-like structure with graphene on the top.
Graphene, a single layer of carbon atoms arranged in a honeycomb lattice, has outstanding electrical properties. This enables it to detect weak electrical signals produced when it encounters biomolecules. While others have done the same thing, they were never able to create large enough graphene surfaces to react reliably with trace biomarkers.
By first creating flower-like carbon structures, Gao increases the surface area the way the leaves on a tree might provide more surface area than the plot of land it grows on. The more surface area there is, the more likely it will react with molecules that are present only in one or two parts per trillion.
When Gao places the sensor on skin, sweat moves through the fluid channels to the sensor—or sensors, as Gao’s technology can have an entire array of them. When the sensor detects a biomolecule, it produces an electrical signal that is transmitted by Bluetooth to either a smartphone app or a medical device.
Powering Bluetooth communications is a hurdle that has stumped many researchers. Most often, other researchers simply have patients wear a rechargeable battery pack that connects to their sensor.
Be the Batteries
Instead of batteries, Gao uses a bioreactor. It’s a type of fuel cell that combines lactate, a chemical generated by muscles, with oxygen to produce electrical power, water, and pyruvate (a common body biochemical).
Others have tried this before, but their bioreactors never produced enough energy. Gao’s group again turned to nanoscience, in this case combinations of graphene, carbon nanotubes, and clusters of platinum-cobalt. This combination increased the surface area of the reactor’s anode—and its ability to react with lactate—more than 3,000 times. It also extended its longevity to several days, from an hour or two.
There are many possible uses for the new sensors. They could, for example, automatically measure how nutrition affects health so people can modify their habits or check for pre-diabetic biomarkers. Sensor could also monitor performance and depression, looking for signs of stress.
Intriguingly, wearable technology may allow physicians to investigate new drugs and eventually tailor medication to individual patients, Gao said. “Many drugs have a very narrow therapeutic window,” he explained. “Too much and they are dangerous, too low and they are not effective. Doctors could use wearable sensors to monitor metabolites in the body and prescribe medication specific for each patient.”
Gao plans to commercialize the technology. He is widening his collaborations, working with other researchers to test wearable sensors for heart failure and chronic pulmonary obstruction. He is also looking at ways to reduce sensor costs by manufacturing them using low-cost methods, such as ink jet printing.
It may not be long before wearable sensors—diagnostic labs on an adhesive bandage—are as common as your Fitbit or Apple Watch.