Seeing is Believing
by Lindsay Borthwick
Brain researchers benefit from the open sharing of new tools and technologies
The Author
Brain researchers are benefiting from the open sharing of new tools and technologies, including open-source microscopes that allow them to visualize the brain’s biology in incredible detail. Edvard Moser and his team at the Kavli Institute for Systems Neuroscience are behind a new, lightweight two-photon miniscope, which they used to map the spatial cells types in the midbrain in mice. The miniscope is also open-source: The researchers provide detailed building instructions in a draft manuscript so that their peers can put the technology to work in their labs, too. Learn more about the miniscope and other new discoveries from researchers at the Kavli Neuroscience Institutes in this research roundup.
Open-source Miniscope
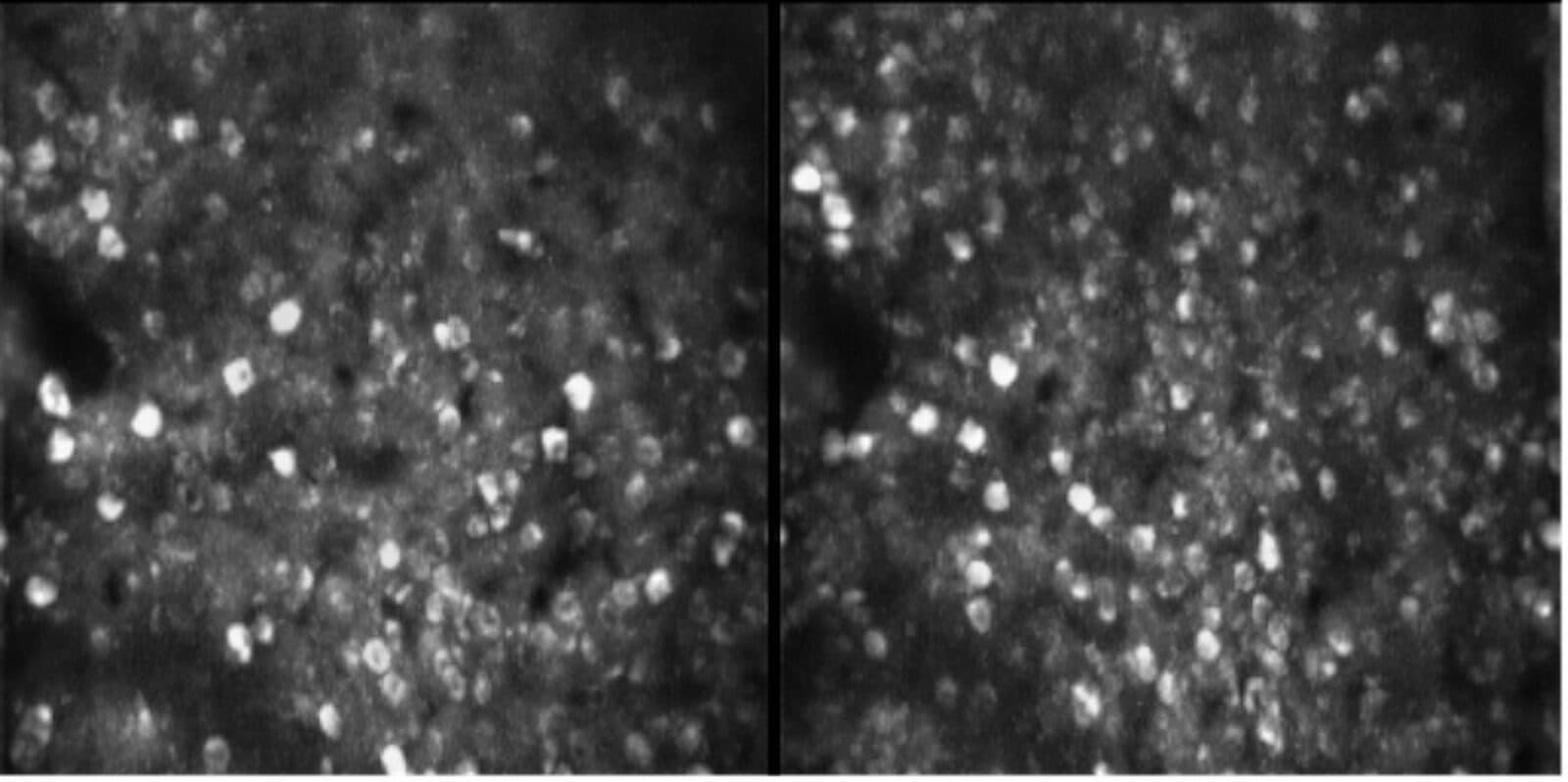
Edvard Moser, scientific director of the Kavli Institute for Systems Neuroscience (KISN) in Trondheim, Norway, and his team recently developed an ultra-light microscope that can capture thousands of neurons at a time in freely moving mice. The miniaturized two-photon microscope, dubbed MINI2P, weighs just 2.4 grams and can be mounted on an animal's head to record its inner workings. MINI2P is the latest evolution of an imaging tool that has transformed neuroscience in recent years by enabling researchers to study the function of individual nerve cells with high precision. In September, the Kavli Institute team posted a preprint, or draft research paper, about the new technology to bioRxiv, along with a second preprint describing how they used MINI2P to map the spatial cell types across the medial entorhinal cortex. The entorhinal cortex is a region that relays spatial information to other parts of the brain, notably the hippocampus. Spatial cells, such as grid cells, place cells and head-direction cells, populate the region. Moser and other scientists have been characterizing those cell types and studying their function for decades, but it is still unclear how those cells are organized. Using the new miniscope to explore the brain's topography at high resolution, the researchers found that grid cells tend to cluster whereas the other spatial cells types intermingle. This arrangement influences how information is processed locally and could validate or inform models for spatial processing in the brain.
We developed a 2.4g miniature multiplane 2-photon microscope recording 1000s of neurons in freely moving 🐭
— Edvard Ingjald Moser (@EdvardMoser) September 21, 2021
and used it to obtain the 1st topographic maps of all spatial cell types.
🇳🇴🇩🇪🇨🇳 @WikiZong and @HorstObenhaus at @KISNeuro, with @trose_neuro, @t_bonhoeffer#KiloNeurons pic.twitter.com/5EXKyyUtnG
Wayfinding for Kids: Teach children about grid cells using this article, "How do we find our way?" penned by May-Britt Moser, scientific director of KISN and a co-author on both preprints. It was published in September in Frontiers for Young Minds, a scientific journal for kids and teens.
Only Human
A pair of studies published in the journal Nature at the end of September shed light on what makes us human. Led by Nenad Sestan of the Kavli Institute for Neuroscience at Yale University, the two studies chart gene expression in the prefrontal cortex (PFC), a brain region implicated in the higher cognitive abilities and complex social behaviors that distinguish humans from other animals. The researchers compared the gene activity patterns in mice, macaques and humans midway through fetal development to find the similarities and differences, then honed in on some of their findings. One study explored the role of retinoic acid, a derivative of vitamin A, which is increased in the PFC during the second trimester—a period when the brain is rapidly expanding and forming synapses in humans. The second study examines the CBLN2 gene, which is also enriched in the PFC. The researchers show that retinoic acid plays a vital role in wiring up the prefrontal cortex during development, activating other genes that together influence where and when new brain connections form. "RA is the first domino to fall, which sets in motion the complex gene networks which lead to development of brain areas associated with human thought," said Kartik Pattabiraman, a co-author of both papers along with Mikihito Shibata and Sestan. Future studies could reveal additional genetic and molecular factors in the brain that are unique to humans.
Song to Speech
What do birdsong and human speech have in common? They are both complex, learned behaviors that may have shared evolutionary roots. That is why researchers at the University of California, San Diego, are studying birdsong on the path to engineering technologies that can restore speech. In the latest study from Vikash Gilja and Timothy Gentner, they recorded local field potentials (LFPs) from the zebra finches and used those signals to predict vocal behaviors, including what syllables the birds would sing and when. Scientists are already using LFPs to predict complex motor behaviors in humans, such as speech, but until now, they have not been studied in detail in birds. "In this paper, we show that there are many similarities in this type of signaling between the zebra finch and humans, as well as other primates. With these signals we can start to decode the brain's intent to generate speech," said Gilja in a news article. The research was funded in part by a grant from UC San Diego's Kavli Institute for Brain and Mind.
Meet the researcher: Read "Translating Birdsong," our recent profile on Gentner and Giilja's research collaboration.
Location, Location, Location!
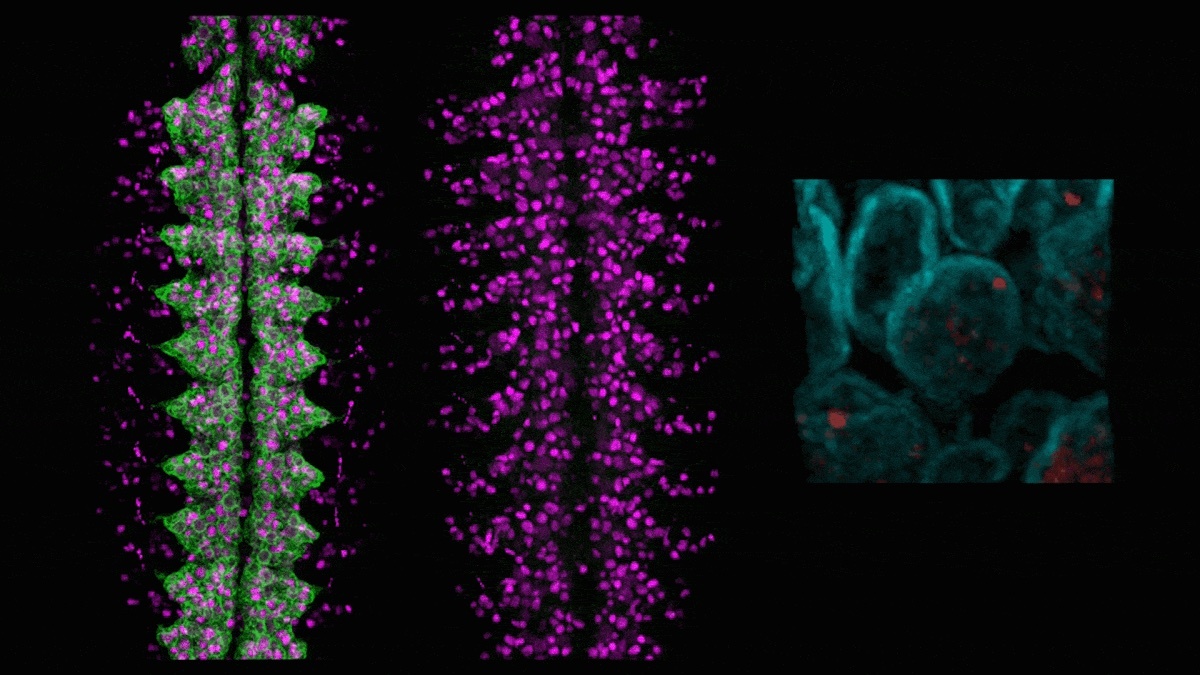
Minoree Kohwi, a member of the Kavli Institute for Brain Science at Columbia University, studies how neural stem cells divide and diversify, eventually forming a complex, healthy brain. In a new study, published in the journal Development Cell, she investigated how the physical location of genes in the nucleus influences cellular diversity in the developing nervous system. It is increasingly clear that the three-dimensional structure of the genome influences which genes are turned on and off during development, which dictates a cell's identity. Working with fruit flies, Kohwi can visualize and track individual genes within the nucleus in developing animals. For example, she can see which genes get relocated to the nuclear lamina, a protein mesh inside most cells where genes are difficult to access and turn on. The new study elegantly describes how a fly gene called hunchback relocates to the lamina after a few cell divisions and how that movement influences the downstream identity of nerve cells in the fly's nervous system. "Similar things might be happening to lots of other genes – some are sent away for permanent storage, while others might be coming out of storage when they're needed at subsequent stages of brain development," she says in a Q&A about the study.

Slowing Alzheimer's
A feature article in Johns Hopkins Magazine highlights a clinical trial for a drug that could slow the progression of Alzheimer's beginning in its earliest stages. The compound, called AGB101, could be a gamechanger for Alzheimer's patients and their families by protecting them from "the horrendous protracted deterioration of a person over the course of the dementia phase," says Michaela Gallagher, a professor of neuroscience and member of the Kavli Neuroscience Discovery Institute. The drug results from a research collaboration between Gallagher and neurology professor Marilyn Albert, both of whom have spent their careers studying the brain and its function, including the cognitive decline and memory loss experienced by some people as they age. Gallagher hypothesized that levetiracetam, an FDA-approved epilepsy drug, could be used to calm hyperactive neurons in the brain during the early stages of Alzheimer's disease. The results of her early research in rodents and humans were compelling. They led to the development of AGB101, a low-dose, time-release version of levetiracetam, which is now in phase 3 clinical trials. Whether their drug succeeds or fails, the researchers are confident that breakthroughs are around the corner that will make Alzheimer's a preventable and treatable condition within a few decades.